X Ray Lead Protective Apron,Lead Protection Aprons,Xray Lead Gown,X Ray Lead Radiation Proof Skirt Longkou Kangxie Medical Instrument Co., Ltd , https://www.sdkangxiemedical.com
These new drugs have been listed: stone medicine, Hengrui, Zhengda Tianqing...
Medical Network July 15th In the past 2018, more than 100 INDs were accepted by the national drug supervision department for a record high in the whole year; in a flash, 2019 has gone through halfway, and the domestic innovative drug category 1 registration has 57 The varieties entered the IND stage and the momentum remained strong.
1. The first half of 2019 ~ the general status of the new drug registration class 1 IND
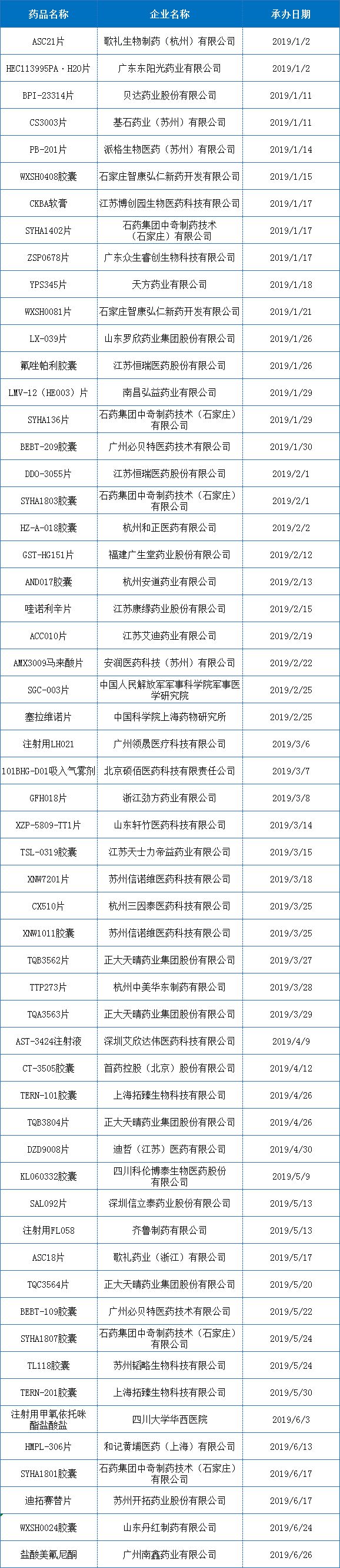
In the first half of 2019, there were about 57 IND varieties registered for domestic Class 1 new drugs (domesticized drugs), and the development situation of innovative varieties was in a growing trend. The month with the largest number of registered applications in the first half of the year was January, and a total of 16 varieties entered the IND. stage.
In terms of enterprises , enterprises with a relatively large number of declarations (≥4 varieties) are Shijiazhuang and Zhengda Tianqing, and other registered enterprises are mostly 1 to 2 varieties entering the IND; it is worth noting that the registration of new drugs in Suzhou is hot. For example, Suzhou Zewei, Suzhou Yasheng, Suzhou Xinnuowei, Suzhou Shuluo, and Suzhou Pioneer, all have 1 or 2 varieties entering the IND stage.
   Figure 1: Distribution of domestic IND registration declarations from January to June 2019
2. Stone medicine & Zhengda Tianqing (≥4 IND varieties)
☆~ stone medicine
In addition to the star variety butylphthalide, the antibody drug conjugate ADC (DP303c) and the humanized gap junction protein 43 monoclonal antibody (ALMB-0166) have obtained the qualification of orphan drugs issued by the US FDA and launched new drugs. In clinical trials, levamlodipine maleate has been applied for NDA in the United States, and the strength of new drug development is sufficient to prove it.
Domestically, in the first half of 2019, the follow-up innovative varieties of Shijiazhuang continued to exert their strength. The number of INDs registered in the first class of new drugs in China was 5, leading to the declaration of new drugs by domestic enterprises; the five varieties were SYHA1402, SYHA136 and SYHA1803, respectively. SYHA1807 capsules, SYHA1801 capsules.
☆~æ£å¤§å¤©æ™´
Zhengda Tianqing, has a new class of new drug isoglycyrrhizinate injection (Tianqing Ganmei), a new class of new drug erlotinib successfully listed, more than 50 innovative drug projects under research, widely praised, and research and development costs more than 10 annual investment One hundred million yuan is one of the pharmaceutical companies with more investment in innovative medicine research in China.
Domestically, in the first half of 2019, Zhengda Tianqing registered 4 domestic INDs of Class 1 new drugs, namely TQB3562, TQA3563, TQB3804 and TQC3564.
3. Brief introduction of the first half of 2019 to the domestic IND variety
Due to various reasons, most of the varieties declared for IND will be kept in a confidential state, and some varieties have not been clinically immediately, and information disclosure will be relatively small. Through further enquiries, the following ten varieties of information can be found online for everyone to learn.
☆~TERN-101
TERN-101, originally developed by Lilly, is a highly effective non-alcoholic FXR agonist for the treatment of nonalcoholic steatohepatitis. In 2018, Takuya Bio and Lilly signed a cooperation agreement to acquire the global development, production and commercialization rights of TERN-101 for the treatment of NASH.
In April 2019, Takuya Biotech published preclinical data at the EASL International Liver Disease Annual Meeting in Vienna, demonstrating that TERN-101 can reduce hepatic steatosis, inflammation and liver fibrosis in a diet-induced obese mouse NASH model; In the same month, NMPA accepted the clinical trial application for this product.
In June 2019, Takubo launched a clinical phase I trial of the FXR agonist TERN-101 to further support the clinical study of TERN-101 for the treatment of NASH.
☆~AST-3424
AST-3424 is a first-in-class anticancer drug developed by Ai Xin Dawei and Taiwan Hao Ding with over-expression of AKR1C3. AST-3424 selectively releases a potent DNA alkylating agent under the action of AKR1C3 to initiate the mechanism of quenching cancer cells; this selective initiation mechanism makes AKR1C3 resistant to multiple refractory cancers, such as liver cancer. Castration-resistant prostate cancer, T-cell acute lymphocytic leukemia and other tumor cells are highly expressed.
Taiwan Hao Ding is conducting a clinical phase I/II study of solid tumors in the United States. In July 2018, OBI-3424 was granted FDA orphan drug status. In April 2019, NMPA accepted the clinical trial application for this product.
☆~TSL-0319
TSL-0319 Capsule is a dipeptidyl peptidase 4 (DPP-4) inhibitor independently developed by Tasly, which inhibits glucagon-like peptide-1 (GLP-1) and glucose-dependent insulin-promoting polypeptide (GIP). Inactivation, promote insulin release from the islets, thereby increasing insulin levels and lowering blood sugar.
The drug belongs to Class 1 chemical drugs and has completely independent intellectual property rights. It is an innovative drug that is not listed at home or abroad. The project has already invested in research and development costs of approximately 169.465 million yuan.
☆~LH021 for injection
LH021 has the potential to repair damaged cartilage of the knee joint and is a drug for improving the condition. In 2015-2016, the mechanism research was completed; in May 2016, the project was officially established; in June, the pharmaceutical CMC was started; in December, the domestic patent was applied; in June 2017, the security evaluation was initiated; in September 2018, the IND package was officially Completed; pre-IND meeting in October; officially submitted IND in January 2019; officially accepted in March.
The idea of ​​clinical trial design will mainly draw on the experience of clinical research on DMOAD drugs abroad. Referring to the clinical trial experience of similar products at home and abroad, LH021 first-time human phase I clinical trials will select patients with primary knee osteoarthritis who meet the inclusion criteria, but not healthy subjects.
Phase I clinical trials, in addition to investigating the tolerance and pharmacokinetic characteristics of the LH021 dose-climbing process, will also explore the relationship between the dose and efficacy of LH021 and the biomarkers associated with cartilage synthesis and metabolism, for the development of a phase II clinical trial dosing regimen. Provide evidence.
The first human clinical trial used a single combined multiple dose escalation for the treatment of primary knee osteoarthritis. After 28 days of single administration, 28 days of follow-up after one cycle of multiple administration, the test period was 11 weeks.
☆~TTP273
TTP273, a non-peptide, highly selective glucagon-like peptide-1 receptor (GLP-1r) agonist discovered by vTv using its small molecule drug discovery platform, is the world's first for vTv (first-in-class) ), the Phase IIb clinical study has been completed in the United States, and the results show that TTP273 has a significant effect on reducing glycosylated hemoglobin in diabetic patients and is well tolerated. The biggest feature and highlight of the TTP273 project is that the currently marketed GLP-1 and its analogues are all injectable, while TTP273 is the first small non-peptide oral dosage form. China and the United States East China introduced the TTP273 project through licensing, and complemented the company's existing LLP-type diabetes product line to complement the company's existing liraglutide injection.
☆~101BHG-D01
101BHG-D01, a new class 1 drug developed for Beijing Shuoyi Medical Technology Co., Ltd. for the treatment of asthma/coagulopathy. Beijing Shuoyi Pharmaceutical Technology Co., Ltd. was restructured in 2016 based on Beijing Fengshuo Weikang Technology Development Co., Ltd. (established in 2001) and Beijing Jiashi Lianbo Pharmaceutical Technology Co., Ltd. (established in 2007). set up.
The company has a total of 40 R & D team, the core team has more than 30 years of experience in the development of new drugs, divided into the Department of Pharmacy, Clinical Medicine, Administrative Personnel, Intellectual Property Department and other departments, the main business is innovative drug research and development.
☆~LH021
LH021, a new class of drugs for the treatment of osteoarthritis independently developed by Guangzhou Lingyi Medical Technology Co., Ltd.
Founded in 2012, Guangzhou Lingyi Medical Technology Co., Ltd. is engaged in the research and development of innovative drugs for new targets. The company was founded by overseas students and elites in the domestic pharmaceutical industry. At present, there are 12 new drug projects in the company's research and development pipeline, covering cancer and digestive diseases. Such as major chronic diseases and specialist diseases related to aging.
☆~GST-HG151
GST-HG151 is a reversible drug for non-alcoholic fatty liver disease and liver fibrosis developed by Fujian Guangshengtang Pharmaceutical Co., Ltd. and Shanghai WuXi PharmaTech New Drug Development Co., Ltd. Preclinical studies have demonstrated the role of improving liver function and significant anti-fibrotic effects, and are expected to fill the gap in the field of global anti-fibrosis and overcome the worldwide problem of irreversible liver fibrosis and cirrhosis.
☆~LX-039
LX-039, a new class of breast cancer developed by Shandong Luoxin Pharmaceutical Group Co., Ltd., is a new, orally effective selective estrogen receptor downregulator that will be used primarily for the treatment of advanced breast cancer. . At present, there is no oral drug for this mechanism at home and abroad, and similar products are in clinical phase I/II.
☆~YPS345
YPS345, a new class of drugs developed by Tianfang Pharmaceutical Co., Ltd. and the Institute of Biophysics of the Chinese Academy of Sciences for the treatment of pneumonia and pulmonary fibrosis caused by thoracic radiotherapy in cancer patients, has no similar products on the market.
Tianfang Pharmaceutical has carried out YPS345 non-clinical trial research stage. The results of efficacy and safety studies show that as a drug for preventing and treating radiation inflammation, it has a significant alleviation effect on radiotherapy-induced pulmonary fibrosis in animal models. And the security is good.
☆~CS3003
CS3003, a selective HDAC6 inhibitor developed for the cornerstone drug, can be used as a monotherapy or in combination with conventional standard therapies, with the potential to demonstrate better efficacy in multiple myeloma.
Preclinical data and preclinical and early clinical studies of similar products have also found that CS3003 may be more safe than broad-spectrum HDAC inhibitors, and may be developed in combination with immunological checkpoint inhibitors in different indications. potential.
☆~ASC21
ASC21, developed by Gyula Pharmaceuticals, is a nucleotide inhibitor that binds to NS5B polymerase and blocks chronic hepatitis C virus infection by inhibiting the activity of NS5B polymerase. Preclinical studies have shown that ASC21 is an effective pan-genotype drug with a high resistance gene barrier.
The ritual plan is used in combination with Ravidavir for the treatment of patients with refractory, cirrhotic and HCV/HIV co-infections.
Schedule: January to June 2019 - Domestic 1 class new drug IND data