First, feed supply Many farmers believe that the mother quail should increase the amount of feed and supplementary foods to increase nutrition after pregnancy. However, many years of practical experience have proven that doing so would be counterproductive. The correct ratio of feed and auxiliary products should be that within 40 days after mating, the number of feedings should be once a day, and the feed amount of each should be generally not more than 35 grams of corn flour or full price feed and 20 grams of fish meal. The maximum amount of eggs is 5 eggs and 1 egg (75 grams). If you feed too much, it is very likely that your baby will be absorbed because of an excess of nutrients. Therefore, farmers who are not very experienced are not able to feed too much daily during this period. After 40 days of pregnancy, the foetus has basically formed, and the feed and auxiliary products can gradually increase. Each two-day increment is 10 grams of flour and 3 grams of fish meal. By the end of the 50th day, the total amount must reach 75-80 grams of flour, 35 grams of fishmeal, and 1/2 egg per day per day (single feed at noon after cooking). After this, two times a day should be fed, once in the morning and once in the evening. Second, drug feeding In addition to the normal rotation of vitamin A, B, C, E and cod liver oil, after 50 days of pregnancy, vitamin C should be added to the feed every day (one dose of water), which can effectively prevent the occurrence of Achilles red claw disease. Regardless of the type of medicine you are feeding, spread it as evenly as possible into the food to avoid remaining. Third, nursing care When the mother-in-law is farrowing, the weather has not yet completely warmed up and the temperature difference between day and night is relatively large. Therefore, the nursing home should be kept warm. First, prepare a straw curtain made of straw. When it is about 1 month pregnant, first cover all the rear litters for the litter, so that the mother has a sufficient period of adaptation. In accordance with the expected date of production, the gates in the middle of the front and rear nests were opened 5 days in advance, so that the calves were familiar with the “birth-room†environment. At the same time, the front door was also covered with a straw curtain to prevent the mother-in-law from panic when the baby was released, which was detrimental to the birth. Before preparing for the above measures, the bottom of the “birth room†must be padded with rice straw or wheat straw for the purpose of heat preservation. Zhang Jiusheng, Qicun Town, Jianggezhuang Town, Leting County, Tangshan City After the mating is completed, the puerpera enters the gestation period. Feeding and nursing at this time are of vital importance throughout the breeding process. Therefore, attention must be paid to it. Otherwise, it may result in loss of cultivation and damage to breeding. Under normal circumstances, the majority of mother-in-law is in gestation during March, and farmers should grasp the following principles to ensure the success rate and birth rate.
Intermediates of Cladribine, Carvedilol, Lurasidone, olmesartan,
Risedronate Sodium, Atazanavir, Saxagliptin,
Dabigatran,Dapoxetine,Cefixime,Ceftaroline fosamil and etc.
In the short span of time, we have emerged as most promising
pharmaceutical intermediates manufacturers, chemical intermediates and
bulk drug intermediates suppliers. Our consistent supply, quality
products and dedication towards clients have opened up many
international avenues for our growth.
In addition, the company also can follow the customer's product needs custom synthesis services
MAIN API PRODUCTS USP/BP
PRODUCT NAME
CAS NUMBER
SPEVIFICATION
Azithromycin
117772-70-0
BEP
Cefpirome Sulphate sterile
84957-29-9
USP JP16
Ceftriaxone Sodium (Sterile)
104376-79-6
USP31
Cefotaxime
64485-93-4
USP30
Ciprofloxacin HCL
85721-33-1
USP/BP
Gentamicin sulphate
1405-41-0
BP
Levofloxacin
100986-85-4
USP27
Lincomycin Hydrochloride
859-18-7
EP6.0
Moxifloxacin Hydrochloride
186826-86-8
USP31
Tigecycline
220620-09-7
USP
Linezolid
165800-03-3
EP
Dexamethasone
50-02-2
USP/BP/EP
Methylprednisolone
83-43-2
USP/BP/EP
Dexketoprofen trometamol
156604-79-4
BP2008
Ibuprofen
15687-27-1
BP
Metamizol
68-89-3
DAB
Sulindac
38194-50-2
USP/BP/EP
Naproxcinod
163133-43-5
USP28
Tripelennamine Hydrochloride
154-69-8
USP28
Itraconazole
84625-61-6
USP/BP
Cytarabine
147-94-4
USP31
Leucovorin Calcium
1492-18-8
USP32
Valsartan
137862-53-4
USP30
Telmisartan
144701-48-4
USP31
Rosuvastatin Calcium
147098-20-2
USP/BP
Pitavastatin Calcium
147526-32-7
USP/BP
Fluvastatin
93957-54-1
USP31
Vinpocetine
42971-09-5
EP6.0
Atazanavir
198904-31-3
BP
Rosiglitazone
122320-73-4
USP30
Esomeprazole Magnesium
161973-10-0
USP/BP
Topiramate
97240-79-4
USP31
Fexofenadine HCl
153439-40-8
Inhouse
Bosentan
147536-97-8
Inhouse
D-Cysteine
921-01-7
Inhouse
D-Phenylalanine
673-06-3
Inhouse
Linagliptin
668270-12-0
Inhouse
Rivaroxaban
366789-02-8
USP
Saxagliptin
361442-04-8
USP
Vildagliptin
274901-16-5
USP
Major Pharmaceutical Intermediates
Mica Ester,Pharma Intermediates,Ciprofloxacin Hcl Uses,Active Pharmaceutical Ingredients NINGBO VOICE BIOCHEMIC CO. LTD , https://www.pharma-voice.com
Items Descripation
Structure
Application
MICA ESTER
CAS No: 246035-38-1
Purity: ≥98%
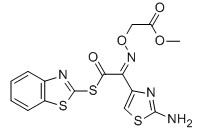
For Cefixime
EHATA
CAS No: 64485-82-1
Purity: ≥98%
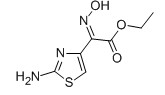
For Ceftazidine
2-Chloroadenine
CAS No: 1839-18-5
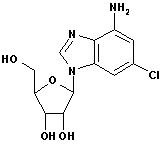
For Cladribine, Fludarabine et al
Bicyclo(2,2,1)Heptane-2,3-di-exo-carboximide
CAS No: 14805o-29-9
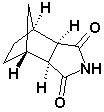
For Lurasidne
(R,R)-1,2-Bis(methanesulfonyloxy methyl)Cyclohexane
CAS No: 186204-35-3
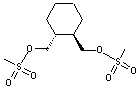
For Lurasidone
3-(Piperazin-1-yl)benzol[d] isothiazole
CAS No: 87691-87-0
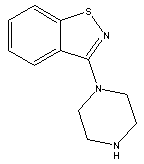
For Lurasidone
Trityl olmesartan
CAS No: 144690-92-6
Purity: ≥98%
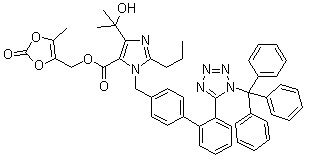
For olmesartan
3-Acetyl Pyridine
CAS No: 350-03-8
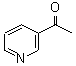
For Risedronate Sodium
3-(AceticAcid)pyridine HCL
CAS No: 6419-36-9
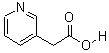
For Risedronate Sodium
Risedronic Acid
CAS No: 105462-24-6
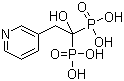
For Risedronate Sodium
3-Hydroxy-1-adamantyl-D-Glycine
CAS No: 709031-29-8
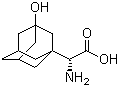
For Saxagliptin
(1s,3s,5s)-3-(aminocarbonyl)-2-azabicyclo(3,1,0) hexane-2-carboxylic acid tert-butyl ester
CAS No: 361440-67-7
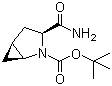
For Saxagliptin
(S)-N-Boc-3- hydroxy-adamantylglycine
CAS No: 361442-00-4
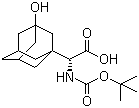
For Saxagliptin
2-Azabicyclo[3.1.0] hexane-3-carbonitrile, (1s,3s,5s)-
CAS No: 866083-42-3
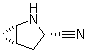
For Saxagliptin
Ethyl 3-(pyridin-2-ylamino) propanoate
CAS No: 103041-38-9
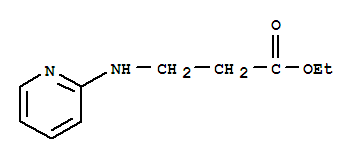
For Dabigatran
N-(4-Cyanophenyl) glycine
CAS No: 42288-26-6
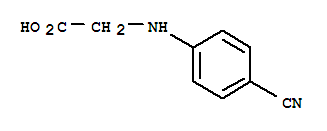
For Dabigatran
4-methylamino-3-nitrobenzoic Acid
CAS No: 41263-74-5
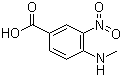
For Dabigatran
S-3-Amino-3-phenylpropanoic acid ethyl ester HCL
CAS No: 167834-24-4
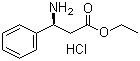
For Dapoxetine
(S)-3-Amino-3-Phemylpropan -1-ol
CAS No: 82769-76-4
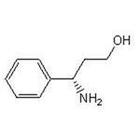
For Dapoxetine
(S)-3-Dimethylamino-3-Phemylpropanol
CAS No: 82769-75-3
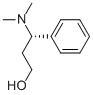
For Dapoxetine
4-{4-[4-(hydroxydiphenylmethyl)-1-piperidinyl]-1-butynil}-α,α-dimethyl benzene acetic acid
CAS No: 832088-68-3
For Fexofenadine HCl
Methyl 2-(4-(4-chlorobutanoyl)phenyl)-2-methylpropanoate
CAS No:154477-54-0
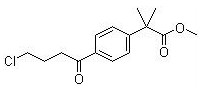
For Fexofenadine HCl
5-Bromo-2-chlorophenyl)(4-ethoxyphenyl)methanone
CAS No 461432-22-4
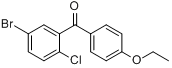
For Dapagliflozin
4-(5-Bromo-2-chlorobenzyl)phenyl ethyl ether
CAS No :461432-23-5
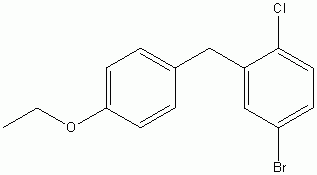
For Dapagliflozin
Pregnancy management tips
Prev Article
Grape autumn clever management