Guide In this paper, datomycin is used as a case to share the challenges and opportunities of current antibiotic APIs, especially the current policies of environmental protection, medical new policy and science and innovation, and the state encourages innovation and squeezes. Under the situation of the marketing expenses of drugs, how do pharmaceutical companies build technical highlands and environmentally friendly highlands? This article provides some useful references and experiences for everyone. Daptomycin is a novel cyclolipopeptide antibiotic obtained from the fermentation of Streptococcus mutans. It not only works on most clinically relevant Gram-positive bacteria, but also methicillin-resistant in vitro. Isolated strains such as vancomycin and linezolid exhibit strong activity, which is of great significance for clinically saving patients with critically ill infections. As early as the end of the 20th century, daptomycin was first developed by Eli Lilly and Company. After 2015, Haizheng Pharmaceutical, Huadong Pharmaceutical, Jiangsu Hengrui, Huabei Pharmaceutical and Zhejiang Pharmaceutical successively obtained CFDA's API production. Approval. According to statistics, the global sales of daptomycin has increased rapidly in recent years. As of 2016, it has reached 1.937 billion US dollars. It is expected that the market size of datomycin in the next few years will show an increasing trend year by year. Daptomycin global sales statistics in recent years Innovative separation and purification processes are effective means to reduce costs and improve economic efficiency. They are also environmentally friendly requirements for reducing environmental waste or chemical emissions. Improve purification efficiency: Since the nature of antibiotics is closely related to its structure, the experimental conditions should be determined according to the stability of the target antibiotics, avoiding the degradation of antibiotics during the separation process, structural transformation, biological activity reduction, etc., and then according to physical and chemical properties, The separation mechanism selects the optimal purification method to achieve higher product purity and yield and a smaller purification cycle, which is the most critical prerequisite for the purification of antibiotics including daptomycin. Increased production efficiency: During the purification process, the soft rubber packing of the traditional agarose or dextran matrix is ​​limited by the flow rate and column pressure, and can not be filled with higher column height, which will greatly reduce equipment utilization and production efficiency. In addition, the traditional soft rubber is easy to form a serious adsorption with the impurity pigment after use, and the regeneration is difficult and can not be used after several times, and the life of the filler is severely limited, which greatly increases the input cost of the enterprise. Due to the high price of daptomycin, which limits its sales growth in the world, especially in China, the maximum cost reduction in the process of R&D and production by pharmaceutical companies will significantly reverse this situation and adopt a new chromatographic separation. Fillers and innovative purification processes to reduce separation and purification costs are undoubtedly key. Environmental protection requirements: The main sources of pollution in industrial separation and purification include waste fillers and mobile phase waste liquids. In order to better meet the stringent requirements of the country in the field of environmental protection, only fillers with longer life and better separation performance can be significantly reduced. The amount of waste material to be disposed of can only greatly reduce the difficulty and cost of subsequent waste liquid treatment by adopting an environmentally friendly mobile phase solution. At present, many enterprises still face major challenges in the above two aspects. The so-called “4+7†drug procurement is to carry out centralized drug procurement in 11 pilot cities, and 31 varieties (42 product specifications) have been identified. The original research or generic drugs that pass the consistency evaluation can participate in the declaration. . The quantity purchase is to promise the sales volume of the medicine at the time of bidding, and it is guaranteed to be used within 8-15 months, that is, “to purchase quantity, to change the priceâ€. It is foreseeable that the next large-volume procurement policy will be pushed to all provinces and cities nationwide, and the number of pharmaceutical products will continue to expand. Therefore, for the majority of pharmaceutical companies, it is more efficient to use more cost-effective production materials and upgrade innovation. Production technology will undoubtedly become the best strategy. Nano has extensive experience in the separation and purification of antibiotics. Based on a comprehensive range of high-performance chromatography products, it has developed a new high-quality solution based on precision monodisperse chromatography, which has been recognized and adopted by many large pharmaceutical companies in China. Nawei Technology can develop purified media and processes suitable for different customer requirements according to different upstream production routes and different impurity profiles of customers, to meet the differentiated needs of customers, and to provide technical training services for customers. 1. Compared with the traditional 3 to 4 step purification process of daptomycin separation, Nano Micro uses the new new technology to optimize and upgrade to 2-step purification, which greatly improves the purification efficiency and recovery rate, significantly reduces production costs and environmental protection investment, and helps enterprises to further improve their products. Market competitiveness and value. 2. Nano's research on vancomycin purification process has also been effective: a large European pharmaceutical company replaced the 13,000 L filler of a well-known Japanese company with 3000 L of monodisperse filler by using the special filler and purification process developed by Nano Micro. The purification efficiency is increased by 4 times, the product purity is increased by 2%, the yield is increased by 10%, the mobile phase is saved by 50%, the waste liquid treatment amount is reduced by 50%, the production cost is greatly reduced, and the production efficiency is greatly improved. 3. Nano's innovation in the purification of Newcombine B0 is reflected in the replacement of disposable amorphous silica filler with recyclable monodisperse silica filler, which greatly improves the purification efficiency and production efficiency, and significantly reduces labor intensity, solvent usage and environmental cost. Wait. Nanotech can provide a full range of chromatography products from HPLC/UPLC analytical packing to large-scale industrial preparation of silica gel, polymer reversed phase, ion exchange, hydrophobic and affinity chromatography, and has a Professional separation and purification overall solution technical team. Up to now, typical examples of the separation and purification of antibiotics successfully developed by Nawei include, but are not limited to, geldanamycin, echinomycin compounds, cyclosporin A derivatives, lincomycin, patroxocin B, Nefing net, teicoplanin, dalbavancin, etc. We look forward to working with you, please feel free to contact us!
Cosmetics are compound mixtures made of various raw materials through reasonable deployment and processing. Cosmetics have a wide variety of raw materials with different properties. According to the raw material properties and uses of cosmetics, it can be roughly divided into two categories: matrix raw materials and auxiliary raw materials.
The former is a kind of main raw material of cosmetics, which occupies a large proportion in cosmetic formulations and plays a major functional role in cosmetics. The latter is responsible for shaping, stabilizing or imparting color, fragrance and other properties to cosmetics, which are not used in cosmetic formulations, but are extremely important. Cosmetics are chemical mixtures made of natural, synthetic or extracted substances with different functions as raw materials and processed through production procedures such as heating, stirring and emulsification.
Our company provides various cosmetic raw materials
Beta Arbutin Powder,Cosmetic Raw Materials,Kojic Acid Dipalmitate Powder,Nicotinamide Mononucleotide Powder XI AN RHINE BIOLOGICAL TECHNOLOGY CO.,LTD , https://www.rhinebiotech.com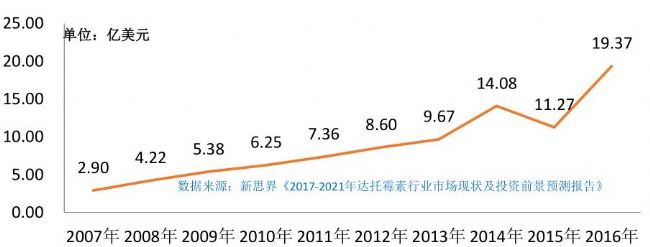
Current challenges in daptomycin purification
"4+7" drug consumption procurement policy forced enterprises to innovate production technology
Nano-assisted antibiotics enterprise optimization and upgrading separation and purification technology 

Under the new medical policy, the road to survival and development of antibiotic raw material medicine enterprises