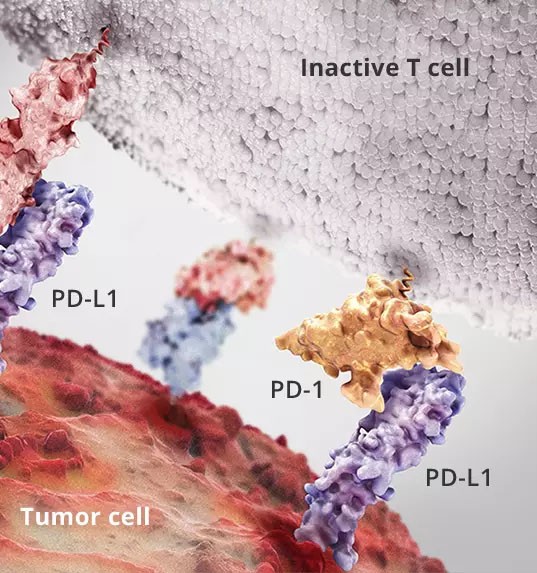
Yesterday, Curis announced that the US FDA has accepted the application for new drug research (IND) for the company's product CA-170. Curis, based in Lexington, MA, is a biotechnology company focused on the research and development of human cancer drugs. CA-170 is a small oral molecule for the targeted inhibition of immunological checkpoint programmed death ligand-1 (PD-L1) and T cell activation of immunoglobulin suppressor V-domain (V-domain Immunoglobulin Suppressor of T-cell Activation, VISTA). Certain human cancer tumor cells express a specific ligand, PD-L1, on the cell surface that binds to the homologous receptor PD-1 present on the surface of immune system T cells. The cell surface interaction between tumor cells and T cells through PD-L1 / PD-1 molecules often leads to inactivation of T cells, so the body cannot produce an effective immune response against tumors. Numerous studies have shown that inhibition of regulation mediated by anti-PD1 antibodies or anti-PD-L1 antibodies can lead to the activation of T cells, which in turn observe anti-tumor effects in tumor tissues. In addition to PD-L1 / PD-1, other cell surface molecules associated with immunosuppression, such as VISTA, have also been discovered. A number of therapeutic monoclonal antibodies that target the PD-1 / PD-L1 interaction have been approved for marketing by the US FDA. In addition to the development of related monoclonal antibodies, the search for oral small molecule compounds that are convenient for cancer patients to target immunosuppressive sites is also a frontier in tumor immunotherapy. Small molecule compounds are able to act through intracellular membranes on intracellular targets, so they are used in a wide range of applications. Secondly, small molecules are chemically modified and often have good bioavailability and compliance, effectively avoiding the decomposition and inactivation of enzymes in the digestive intestinal tract. Finally, in the production process, formulation design and mode of administration, small molecule research is also quite mature. Curis's new drug, CA-170, is designed as an oral small molecule drug that selectively targets immune activation. Preclinical data indicate that CA-170 can effectively induce the proliferation of immune T cells and the production of the cytokine IFN-γ (gamma interferon) by inhibiting PD-L1 or VISTA in cell culture experiments. In addition, CA-170 has been shown to be toxicologically safe in multiple mouse tumor models. Dr. Ali Fattaey, President and CEO of Curis, said: "The FDA's acceptance of the IND application for CA-170 marks an important milestone in the field of cancer immunotherapy. Over the past few years, the successful development of multiple immunological checkpoint inhibitors And the market has greatly promoted the effective treatment of many human cancers. Today, the FDA has approved us to test the first small molecule inhibitor, CA-170, which will be suitable for oral treatment of cancer patients. Considering the pharmacokinetic properties of small molecules The many advantages that will demonstrate dose flexibility in monotherapy or other combination regimens. We believe that the successful development of CA-170 will provide patients and doctors with a compelling treatment option." high quality Medical raw material powder products Professional supplier
Medical raw material powder,Posaconazole Pharmaceutical API,Piracetam Pharmaceutical API,Nystatin Active Ingredients,Pure Ranitidine HCL Powder
China leading manufacturers and suppliers of Apis Raw,Copper Peptide Ahk-Cu Powder, and we are specialize in Paracetamol Dc96 Injection,Amlodipine Besylate Powder, etc.
For customer`s needs, OEM service is also acceptable. If you have a good idea in new product production but lack of laboratory device and human resource, we are glad to solve this problem for you. Sincerely hope to strengthen exchanges and cooperation with friends from both home and abroad.
Xi'an Hollysince Biotech Co., Ltd., located in Weiyang District, Xi'an, Shaanxi, China, is a professional researcher and one of the leading Medical raw material powder,Posaconazole Pharmaceutical API,Piracetam Pharmaceutical API,Nystatin Active Ingredients,Pure Ranitidine HCL Powder Xi'an Hollysince Biotech Co., Ltd. , https://www.hollysince.com
manufacturer in the field of Organic extract and high quality Food Supplement.
We are a 12-year manufacturer from China with ISO certification, 320 employees, export products to about 85 countries all over the world. We also have strong professional research and Analysis team. Therefore we have ability to offer you the batch test results to guarante the quality. At the same time, we insist in innovation and own our factory, so we have power to produce and design for you id the quantity you need is large enough You could trust us, we will be your the best optionsDon`t hesitate to communicate with us, waiting for you always .
US first small molecule immune checkpoint inhibitor IND approved
Next Article
ELISA experiment considerations