On June 22, 2018, Beijing Aikang Yicheng Medical Equipment Co., Ltd. (hereinafter referred to as Ikang Medical ) received a written notice from the US Food and Drug Administration (FDA): A3 total knee system has passed FDA510(k) certification. A breakthrough in the FDA certification of domestic knee joint products. The A3 total knee system includes a femoral component, a tibial plate component and a spacer, and a tibial component. It is suitable for patients with end-stage joint disease caused by various reasons and needs knee joint replacement, including osteoarthritis, osteonecrosis, rheumatoid arthritis and post-traumatic arthritis. The A3 total knee system is cemented to replace the articular cartilage of the patient's lesions, eliminate pain symptoms, correct knee deformities, and improve and restore the knee function of the patient. As a pioneering enterprise in the field of bone and joint in China, Aikang Medical always regards the quality of its products as the foundation of its development and always regards the inferior image of domestically produced products as its vision. It can realize the breakthrough of domestic knee joint products in the US FDA certification, which is a strong proof of the pursuit of high quality products by Ikang Medical. The A3 knee joint product has passed the FDA certification, which indicates that the product design concept and quality of Aikang Medical have been recognized by the FDA, which marks that Aikang Medical has entered a big step in the international market and further developed the international market for Aikang Medical. Provides a strong competitive edge. It can bring new profit growth points to the company and create greater value for the majority of shareholders. The FDA is the English abbreviation of the U.S. Food and Drug Administration, which is the authority for international medical audits. Authorized by the federal government, the highest law enforcement agency specializing in food and drug management. In the United States and many other countries, only FDA-approved materials, devices, and technologies are available for clinical commercial applications. Since 1990, the US FDA and ISO and other international organizations have closely cooperated to continuously promote innovative measures. Especially in the medical field, FDA certification has become the highest inspection standard in the world medical field, and has been recognized by WHO as the highest safety standard. The state promotes and monitors the safety of domestic products by seeking and receiving FDA's help. Many international manufacturers also obtain FDA certification as the guarantee and highest honor for product quality. D-tagatose is a rare natural monosaccharide, which is the ketose form of galactose and the epimer of fructose. The sweet characteristic is similar to sucrose, and the heat produced is only one third of sucrose, so it is called low calorie sweetener. Tagatose has excellent nutritional characteristics such as low caloric value, zero glycemic index, blood glucose passivation, caries free, prebiotic effect and Antioxidant activity. Tagatose has been approved by FDA as a pharmaceutical excipient for throat moistening tablets, over-the-counter diabetes cough syrup, chewable antibiotic tablets, non steroidal anti-inflammatory drugs and pediatric drugs. FDA Approves tagatose to replace sucrose and other sweeteners in toothpaste, mouthwash and cosmetics. D-Tagatose Powder,Aspartame Powder,Erythritol Powder,Monk Fruit Extract Xi'an Tian Guangyuan Biotech Co., Ltd. , https://www.tgybiotech.com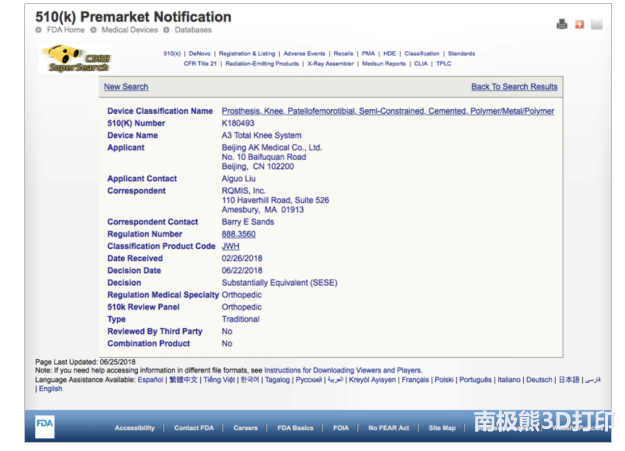

Aikang Medical A3 Total Knee System is FDA certified
Next Article
Pea planting time and planting points