Release date: 2018-06-29 On June 27th, CNDA approved the IND application for the JGCAR029, a charcoal-based CAR-T product, which is the first clinically approved CD19-targeted CAR-T product in China. Taking this opportunity, the author simply combed the current clinical trials of CAR-T therapy. CAR-T cell immunotherapy (chimeric antigen receptor T cell immunotherapy) is one of the most disruptive emerging technologies in the field of cancer therapy, compared with other tumor treatment methods, such as surgical resection, radiotherapy and chemotherapy, small molecule targeting. Drugs, monoclonal antibody drugs, and hematopoietic stem cell transplantation have many advantages such as more "precision", more "flexibility", more "broad spectrum", and more "lasting". Currently, two CAR-T cell immunotherapy products have been approved worldwide, namely Novartis KymriahTM and Kite Pharma's Yescarta. Globally, the number of clinical trials of CAR-T therapy is increasing dramatically. According to the statistics of the Cancer Research Institute (CRI) [i], as of February 2018, a total of 404 CAR-T projects worldwide are in clinical trials, mainly headed by China and the United States. Among them, there are 171 in the United States in clinical trials, and 152 in China. China and the United States account for 79.95% of the national CAR-T cell therapy, becoming the world's two giants of CAR-T cell immunotherapy. Number distribution of CAR-T clinical trials in different countries and regions (Source: ClinicalTrial) At present, the CAR-T project in clinical research involves more than 47 targets. From the distribution of target sites, clinical trials of CAR-T cell therapy mainly focus on CD19, CD20, CD22, GPC3, BCMA and other popular targets. From the distribution of CAR-T therapy targets in the United States, CD19-targeted clinical trials accounted for more than 40%. Previously, two CAR-T products approved by Novartis and Kite Pharma were also targeted at CD19. point. Followed by BCMA, CD22, CD30 and other targets. Similar to the distribution of target sites in the United States, the number of CAR-T clinical trials targeting CD19 in China is also over 40%. However, the number of CAR-T clinical trials in the United States is second only to the popular target BCMA of CD19, while the number of clinical trials in China is less than that of CD20, CD22 and GPC3, accounting for only 5%. According to statistics, as of the end of May 2018, 17 of the 22 CAR-T project applications accepted by CDE were CD19 target projects, while the number of projects targeting BCMA was only 4, respectively. LCAR-B38M cell preparation of legendary organism (currently obtained clinical approval for domestic drugs), CT053 whole human anti-BCMA autologous CAR-T cell injection of Shanghai Keji Pharmaceutical Co., Ltd., anti-human of Shanghai Hengrun Dasheng Biotechnology Co., Ltd. BCMA T cell injection, Shanghai Youkadi Biological Programmed Death Receptor 1 knockdown targeting CD269 chimeric antigen receptor engineered T cell injection. The United States and China are investigating the distribution of CAR-T targets (Source: ClinicalTrial) The target determines the direction of the indication. In terms of indications, CAR-T cell immunotherapy has been proven to have made breakthroughs in the treatment of hematological malignancies, showing good targeting, lethality and persistence. The two CAR-T products approved by Novartis and Kite Pharma are also suitable for hematological tumors, which are suitable for acute lymphoblastic leukemia and B-cell lymphoma, and are still expanding to other hematological tumors, such as follicular lymphoma, Cell tumors, multiple myeloma, etc. CAR-T is currently making relatively slow progress in solid tumor applications. Statistics show that about 75% of the CAR-T clinical trials in the world are mainly used for blood tumors such as leukemia and lymphoma. Only a small part of the CAR-T project is for solid tumors such as liver cancer and lung cancer. CAR-T progresses significantly on hematological tumors and is slow in solid tumors. On the one hand, there are large differences in the traits of solid tumors and blood tumors. Highly heterogeneity makes CAR-T therapy in solid tumors. The difficulty of treatment has greatly increased, and the choice of target has become one of the major challenges faced by CAR-T in the treatment of solid tumors. On the other hand, solid tumor-associated tumor antigens are not only expressed in tumor tissues, but also in normal tissues. The antigen even maintains the normal physiological function of the tissue, how to improve the recognition specificity of CAR-T cells, and reduce the possibility of CAR-T cells attacking normal tissues, which becomes another major challenge for CAR-T to treat solid tumors; The homing and activation maintenance of CAR-T cells in solid tumors is also one of the challenges. How to accurately homing CAR-T cells transported by peripheral blood to tumor sites and function. In the future, with the discovery of more targets and the clear mechanism of action, as well as the development of relevant clinical trials, the application of CAR-T in solid tumors has broader development prospects. From the perspective of market size, taking the domestic example as an example, in the field of hematological cancer, 88,000 new lymphoma patients are newly added each year, 30% of which will develop relapse and refractory; 75,000 new leukemia patients are added each year. About 60% of them will develop relapse and refractory; 3,200 new patients with myeloma each year, almost all of them are BCMA positive, 70% of which will develop relapse and refractory. According to the market penetration rate of 30% (pessimistic)-50% (optimistic), it is estimated that there are about 28,200 to 47,100 blood tumor patients undergoing CAR-T cell therapy every year in China, according to CAR-T therapy pricing 30 - $450,000, the market for domestic blood tumor CAR-T therapy is estimated at $8.4 billion, and is expected to reach $21.2 billion as market penetration increases. With the continuous improvement of the treatment technology for solid tumors, if CAR-T can achieve breakthroughs in solid tumors, the current volume of new human solid tumor patients in China is 3.6 million, according to the proportion of 10% treatment, 30-45 million The treatment price of the US dollar is expected to exceed the market space of domestic solid tumor CAR-T therapy by more than 100 billion US dollars, which will be five times the market space of the blood tumor. It can be seen that the market application of CAR-T in solid tumors is broad. Reference material On May 24, 1.2018, the article "The global landscape of cancer cell therapy" published by Cancer Research Institute (CRI) in Nature Reviews Drug Discovery, New York, USA. Source: Flint Creation Fluorescence Microscope ,Epifluorescence Microscopy,Light Sheet Fluorescence Microscopy,Fluorescent Light Microscope Ningbo ProWay Optics & Electronics Co., Ltd. , https://www.proway-microtech.com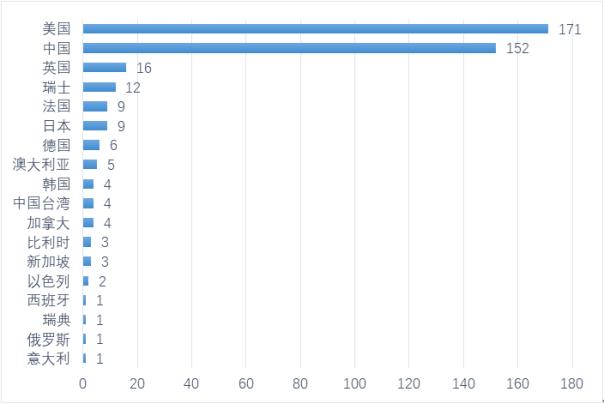
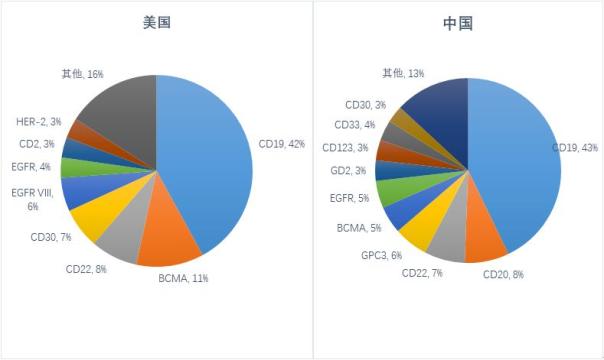
Global CAR-T cell therapy clinical trial situation
Next Article
Tea microwave curing technology
Prev Article
Apple sets up the bag, management do not slack