Medtronic recently announced that its Melody(TM) transcatheter pulmonary valve has been certified by the US Food and Drug Administration (FDA) for implantation in patients with prosthetic failure of pulmonary heart valves, especially Pulmonary valve position. This is the first product of this type to be certified by the US FDA. When a surgical valve that has been implanted in the body gradually ages and fails over time, the patient often needs a new valve for replacement, and the replacement requires a new happy operation. To extend the time interval between two happy operations in patients with functional disorders of the right ventricular outflow tract caused by congenital heart disease (CDH), Melody (TM) can provide a minimally invasive treatment option for these patients. More than 10,500 patients worldwide are now implanted with transcatheter heart valves. The Melody(TM) transcatheter pulmonary valve was first CE certified in September 2006 and began clinically treating pulmonary valve damage. In 2010, Medtronic began to FDA certification. From 2006 to the present, in the past decade, three clinical studies from Medtronic have shown that this product is significantly effective in delaying the interval of open surgery. pulse oximeter Shandong Zhushi Pharmaceutical Group Co.,LTD , https://www.sdzs-medical.com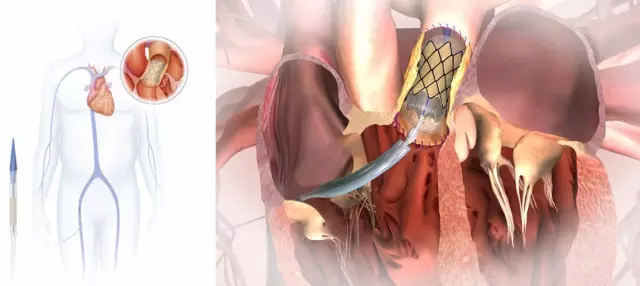

FDA Passes Medtronic Transcatheter Pulmonary Valve Certification
Next Article
Greenhouse peppers are afraid of epidemic diseases
Prev Article
Potato noodle processing technology